“I just can’t make it tonight. You have fun without me.” Across much of the animal kingdom, when infection strikes, social contact shuts down. A study headed by researchers at The Picower Institute for Learning and Memory of MIT has now detailed how the immune and central nervous systems implement this sickness behavior. The scientists and their collaborators used multiple methods to demonstrate causally that interleukin-1 beta (IL-1β) directly modulates activity of IL-1 receptor 1 (IL-1R1)-expressing neurons in the brain’s dorsal raphe nucleus (DRN), activating connections with the intermediate lateral septum to shut down social behavior.
“Our findings show that social isolation following immune challenge is self-imposed and driven by an active neural process, rather than a secondary consequence of physiological symptoms of sickness, such as lethargy,” said study co-senior author Gloria Choi, PhD, associate professor in The Picower Institute and MIT’s Department of Brain and Cognitive Sciences.
The team, headed by co-senior author Choi, together with co-senior author Jun Huh, PhD, Harvard Medical School associate professor of immunology, and first author Liu Yang, PhD, a research scientist in Choi’s lab, reported on their findings in Cell, in a paper titled “IL-1R1-positive dorsal raphe neurons drive self-imposed social withdrawal in sickness,” in which they concluded “… our findings implicate IL-1β as a primary effector driving social withdrawal during systemic immune activation … Our investigation into IL-1β expands upon growing evidence that cytokines serve as neuromodulators within behavior-relevant neural circuits to orchestrate adaptive responses during inflammatory conditions.”
It makes perfect sense that when we’re battling an infection we lose our desire to be around others. This lets us get much needed rest but also protects others around us from getting sick. “Across the animal kingdom, infections often elicit social withdrawal—an adaptive behavioral response believed to preserve the overall health of the group by isolating the sick individuals and thereby reducing the risk of pathogens being transmitted to others,” the team wrote. What hasn’t been as clear is how this behavior change happens. “… it remains largely unexplored whether social withdrawal arises from self-imposed isolation by the sick individual or avoidance by healthy conspecifics and how such behavioral adaptations are orchestrated by the nervous system,” the investigators noted.
There is a close connection between the nervous system, which regulates behavior, and the immune system, which detects pathogens and mounts protective responses during infection, the team pointed out. There is also growing evidence indicating that cytokines—soluble factors expressed by peripheral immune cells—serve as a molecular link between the immune and nervous systems.
Choi and Huh’s long collaboration has identified cytokines that affect social behavior by latching on to their receptors in the brain. For their newly reported study the team hypothesized that the same kind of dynamic might cause social withdrawal during infection. But which cytokine? And what brain circuits might be affected?
To identify which cytokines might act centrally to mediate social withdrawal, the scientists carried out a behavioral screen in mice. They injected 21 different cytokines, one by one, into the animals’ brains, to see which, if any of the cytokines triggered social withdrawal responses similar to those resulting after administration of lipopolysaccharide (LPS), which is a standard way of simulating infection.
Using a modified sociability assay to investigate behavior the researchers found that only IL-1β injection fully recapitulated the same social withdrawal behavior as did LPS administration. That said, IL-1β also made the mice more sluggish. “Among the cytokines tested, only IL-1β fully recapitulated the behavioral effects of LPS …” they stated. “This observation is consistent with previous studies implicating IL-1β in sickness-associated reduction of social exploration.” In contrast, they reported, IL-1α induced hypoactivity but without affecting sociability. “Thus, of the 21 cytokines tested, IL-1β uniquely elicited robust changes in social behavior.”
IL-1β affects cells when it hooks up with the IL-1R1, so the team next went looking across the brain for where the receptor is expressed. They identified several regions and examined individual neurons in each. The dorsal raphe nucleus stood out among regions, both because it is known to modulate social behavior and because it is situated next to the cerebral aqueduct, which would give it plenty of exposure to incoming cytokines in cerebrospinal fluid.
The team’s experiments identified populations of DRN neurons that express IL-1R1 (IL-1R1DRN neurons), including many involved in making the crucial neuromodulatory chemical serotonin. Collective findings from experiments, they noted, “… indicate that IL-1R1 is predominantly expressed in the serotonergic subset of DRN neurons.”
From there, Yang and the team demonstrated that IL-1β activates those neurons, and that activating the neurons promotes social withdrawal. Moreover, they showed that inhibiting that neural activity prevented social withdrawal in mice treated with IL-1β, and they showed that shutting down IL-1R1 in the DRN neurons also prevented social withdrawal behavior after IL-1β injection or LPS exposure. “Our data indicate the presence of a neural mechanism underlying voluntary social disengagement in sick animals and reveal a function of IL-1R1 signaling in dorsal raphe neurons in regulating social behavior in response to systemic immune activation,” the investigators stated.
Notably, these experiments did not change the lethargy that followed IL-1β or LPS, helping to demonstrate that social withdrawal and lethargy occur through different means. “We observed that inhibiting IL-1R1DRN neurons reversed social withdrawal without alleviating hypoactivity,” they stated.
With the DRN identified as the site where neurons receiving IL-1β drove social withdrawal, the next question was what circuit they affected that behavior change through. The team traced where the neurons make their circuit projections and found several regions that have a known role in social behavior.
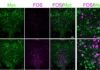
![A figure from the paper illustrates that among cells expressing myc (green), a proxy for the IL-1R1 receptor, neural activation is much greater as measured with fos (magenta) when IL-1 beta was administered vs. when a control (vehicle) chemical was. [Cho Lab/MIT Picower Institute]](https://www.genengnews.com/wp-content/uploads/2025/11/Low-Res_Choi-Figure3B-300x178.jpg)
Using optogenetics, a technology that engineers cells to become controllable with flashes of light, the scientists were able to activate the DRN neurons’ connections with each downstream region. Only activating the DRN’s connections with the intermediate lateral septum caused the social withdrawal behaviors seen with IL-1β injection or LPS exposure.
In a final test, they replicated their results by exposing some mice to Salmonella. “Collectively, these results reveal a role for IL-1R1-expressing DRN neurons in mediating social withdrawal in response to IL-1β during systemic immune challenge,” the researchers stated. “Taken together, our findings support the conclusion that social isolation following immune challenge is self-imposed and driven by an active neural process involving IL-1R1DRN neurons in response to IL-1β, rather than a secondary consequence of physiological symptoms of sickness or social exclusion.”
Though the study revealed the cytokine, neurons, and circuit responsible for social withdrawal in mice in detail and with demonstrations of causality, the results still inspire new questions. One is whether IL-1R1 neurons affect other sickness behaviors. Another is whether serotonin has a role in social withdrawal or other sickness behaviors. “Further studies are required to elucidate how social structures reorganize during prolonged infection and whether early-stage withdrawal effectively mitigates pathogen transmission and enhances overall group resilience,” the investigators further stated.
The post Mapping the Neural Mechanism Behind Social Isolation Following Immune Challenge appeared first on GEN – Genetic Engineering and Biotechnology News.