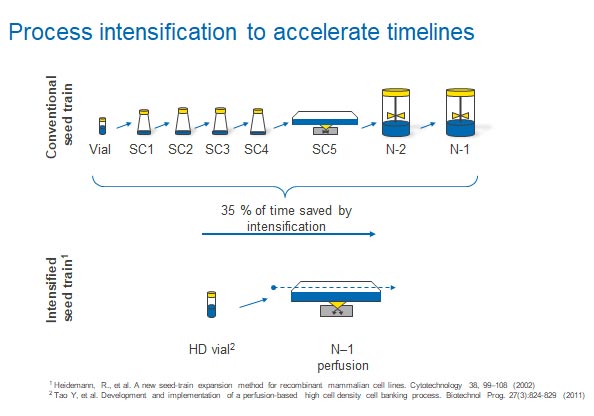
Rentschler Biopharma sped up the cell culture step of its biomanufacturing process by 35% by “seeding” the bioreactor with a larger number of cells. That’s according to Johannes Wirth, a process engineer at the company.
“What’s new is putting it all together in an already-running industrial process, and making this process much better,” he says.
Before the implementation of the intensified process, the company ran a typical fed-batch process, according to Wirth. This often started slowly, with a relatively unproductive phase where cell densities grew slowly, which reduced overall productivity and increased timelines.
To improve performance, the company decided to intensify the seed train phase to generate more cells that could be used as a starting culture in the bioreactor.
“We were able to cut out the unproductive phase by using biomass generated in the intensified seed train,” explains Wirth. “We put this biomass into the production process, and it shortened that as well.”
He went on to explain the greater biomass in the bioreactor meant that cells grew and consumed nutrients more quickly than normal. “So, we adjusted the feeding dynamically, taking the higher growth into account, and were able to deal with the higher cell densities.”
As a third step, the company decided to implement real-time monitoring of the bioreactor using spectroscopic methods. By placing a probe directly into the bioreactor, rather than having a person taking out samples, the risk of contamination was reduced.
 According to Wirth, the company is implementing the intensified seed train and bioreactor seeding into routine cGMP manufacturing while working on multiple client projects in bioprocess development. Implementation of real-time monitoring in routine cGMP manufacturing will follow in due course.
According to Wirth, the company is implementing the intensified seed train and bioreactor seeding into routine cGMP manufacturing while working on multiple client projects in bioprocess development. Implementation of real-time monitoring in routine cGMP manufacturing will follow in due course.
“The new thing is that we’re using this in industrial processes, which are relevant to our industrial clients,” says Wirth, who explains that, although the concept has already been widely discussed in the literature, they’ve typically been tested during academic studies, which have lower productivities.
The company aims to use this intensified approach at Rentschler Biopharma’s manufacturing site in Milford, MA, as well, which was acquired in 2019.
The post Rentschler Biopharma Focuses on Reducing Timelines with Process Intensification appeared first on GEN – Genetic Engineering and Biotechnology News.











