Genetic and fossil analyses are the major methods that have governed studies in evolution and unearthed valuable information on how species change over time. But neither genetic nor fossil analysis can be applied to study the evolution of the brain. The brain tissue is too soft and degrades too quickly for the slow processes of fossil formation. And without physical remnants from which DNA can be extracted, genetic analysis is not possible.
Adopting a novel approach, Alysson R. Muotri, PhD, professor at the University of California (UC, San Diego, School of Medicine, used “mini-brains” or brain organoids derived from stem cells, that serve as a model system for tracing the evolution of Neanderthal and Denisovan brains.
Muotri and colleagues have pioneered the use of stem cells to compare humans to other primates, such as chimpanzees and bonobos. But a comparison of modern humans with extinct predecessors using mini-brains grown from stem cells has not been attempted before now.
Muotri’s team published the findings in an article in Science titled, “Reintroduction of the archaic variant of NOVA1 in cortical organoids alters neurodevelopment,” where they cataloged the differences between the genomes of different modern human populations and the Neanderthals and Denisovans, who lived during the Pleistocene Epoch, approximately 2.6 million to 11,700 years ago.
In the study, the scientists first compared modern human genetic data from the 1000 Genomes Project and the Simons Genome Diversity Project (SGDP) with Neanderthal and Denisovan genomes to identify genetic changes that result in protein-coding differences between the modern human genome and Neanderthal and Denisovan genomes.
Sixty-one genes differed between modern humans and our extinct relatives. One of these altered genes—NOVA1—caught Muotri’s attention because it’s a master gene regulator. NOVA1, short for neuro-oncological ventral antigen 1, is an evolutionarily conserved splicing regulator—a gene that regulates the production of multiple proteins from a single gene, during early brain development.
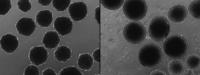
The researchers then used CRISPR gene editing to engineer modern human stem cells with the archaic Neanderthal version of the NOVA1 gene and added inducers to the growth medium to coax the stem cells to specialize into neurons and ultimately Neanderthal-ized mini-brains.
“It’s fascinating to see that a single base-pair alteration in human DNA can change how the brain is wired,” said Muotri, senior author of the study and director of the UC San Diego stem cell program and a member of the Sanford Consortium for Regenerative Medicine.
“We don’t know exactly how and when in our evolutionary history that change occurred. But it seems to be significant and could help explain some of our modern capabilities in social behavior, language, adaptation, creativity, and use of technology,” said Muotri.
Mini-brains or brain organoids are tiny tight clusters of brain cells formed from stem cells, but they aren’t exactly brains. They lack connections to other organ systems, such as blood vessels, separation into specialized regions such as the cortex or the cerebellum, and the structural and functional complexities of real brains.
Yet brain organoids are useful laboratory models for studying genetics, disease development, and responses to infections and therapeutic drugs. In past studies, Muotri and his team have optimized the process of generating brain organoids to achieve periodic electrical waves similar to human brain waves.
The team found that modern and Neanderthal brain organoids differ in the way their cells multiply and how their neurons connect at synapses. They also found a different set of proteins at the synapses when they compared modern and Neanderthal brain organoids.
Analyzing gene expression data, the researchers found 277 genes that were differently expressed in the archaic and modern brain organoids at timepoints analogous to different stages in the neurodevelopmental process.
The study reveals some genes were differently put together during the decoding process to form proteins with different functions in the archaic and modern brain organoids, suggesting that the single base-change results in functional consequences.
Neanderthal brain organoids show higher electrical activity at earlier stages of maturation compared to modern brain organoids, but their electrical activity patterns are not synchronized in networks. Muotri explained that the neural network changes in Neanderthal brain organoids parallel the way newborn nonhuman primates acquire new skills more rapidly than human newborns.
“This study focused on only one gene that differed between modern humans and our extinct relatives. Next, we want to take a look at the other 60 genes, and what happens when each or a combination of two or more are altered,” said Muotri.
“We’re looking forward to this new combination of stem cell biology, neuroscience, and paleogenomics. The ability to apply the comparative approach of modern humans to other extinct hominins, such as Neanderthals and Denisovans, using brain organoids carrying ancestral genetic variants is an entirely new field of study.”
Muotri and co-author and collaborator Katerina Semendeferi, PhD, professor of anthropology at UC San Diego, plan to integrate studies on stem cell-derived organoid models with anatomic comparisons in different species, under different neurological conditions to understand brain function of extinct human predecessors.
“This neuro-archealization approach will complement efforts to understand the mind of our ancestors and close relatives, like the Neanderthals,” said Semendeferi.
The post Archaic Allele Alteration Spells Functional Consequences for our Species’ Evolution appeared first on GEN – Genetic Engineering and Biotechnology News.