“Believe it or not, there was an explicit strategy to have multiple product candidates to de-risk the delivery issue.”
That is what Verve Therapeutics CEO Sekar Kathiresan, MD, told GEN Edge regarding his conviction that delivery was and remains the most pressing problem with in vivo gene editing since the company was initially conceptualized in 2016.
“We knew what we wanted to do because the targets emerge from the human genetics work that we and others did over the years on the genetics of coronary disease resistance factors,” said Kathiresan. “We had a list of targets, with the top one being PCSK9. Then we said, let’s think through the risks here. When we made the list of risks back in 2018, top of the list was delivery.”
On Tuesday this week, Verve disclosed that it had temporarily halted the Heart-1 trial to assess VERVE-101’s safety and tolerability, as well as its pharmacokinetics and changes in blood PCSK9 protein and LDL-C. This followed the discovery of a grade 3 serious adverse event (SAE) in one of the trial participants. Kathiresan emphasized that the patient—one of six who received a higher dose of VERVE-101 and experienced transient reversible changes—has recovered and has been discharged from hospital.
VERVE-101 was designed for patients with high-risk, heterozygous familial hypercholesterolemia (HeFH), established atherosclerotic cardiovascular disease (ASCVD), and uncontrolled LDL-C levels on oral standard-of-care therapy. In this single ascending-dose study, the main purpose was to identify a dose that is safe, efficacious, and tolerable.
After a steep drop of about 40 percent in the share price on April 2 following the news, Verve appears not to have disappeared into the night (a la Graphite Bio) but instead lives to fight another day behind other pipeline leads, VERVE-102 and VERVE-201, which both use a different lipid nanoparticle (LNP) system than VERVE-101.
That’s significant given that Kathiresan insists it was neither the guide RNA nor the base editing process that caused the SAE. There are three parts of the drug—the single-guide (sg) RNA, the mRNA encoding the base editor, and the LNP, Kathiresan explains. Two of the three are working as designed—the guide and the editor. The culprit, Kathiresan believes, is the LNP.
Liver LNP-delivery is not solved
For context, Kathiresan says the seemingly wide-spread view that LNP delivery to the liver is solved is incorrect. “There’s been this assumption over the 12–18 months that liver delivery is solved. I don’t think that’s the case,” said Kathiresan told GEN Edge.
Prior to VERVE-101, there had been one successful example of in vivo gene editing in humans from Intellia—that’s it. Though many research articles and reviews make the claim that in vivo delivery of gene editing tools to the liver is straightforward, most of those studies were done in rodents. Part of the issue lies in how the term LNP is thrown around.
“What’s underappreciated is that there’s just a general term, LNP,” said Kathiresan. “But there’s no general LNP—it’s the chemical matter of a shell.”
Typically, LNPs have four essential components: ionizable cationic lipids, phospholipids, cholesterol, and PEG lipids. The key component is the ionizable cationic lipids (sometimes called aminolipids); each one has its own chemical structure with unique properties that influence both safety and efficacy that vary with the organism being targeted (e.g., the same LNP that worked to target the liver in mice does not necessarily translate to NHPs and humans).
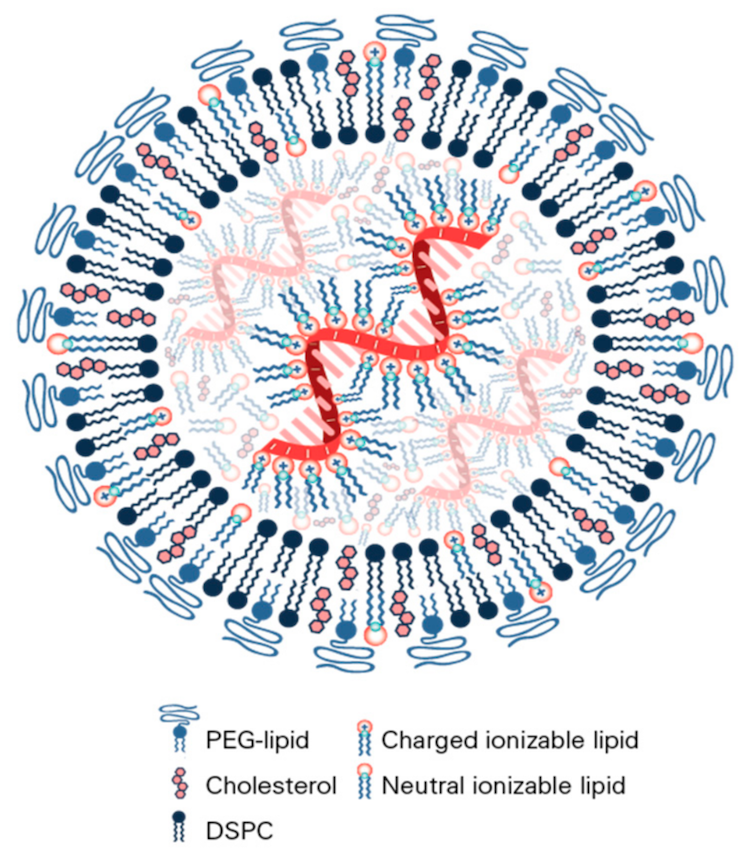
To create an LNP packaged with gene editing cargo, there are a couple of approaches to bringing the negatively charged nucleic acids—the sgRNA and mRNA—with the lipids and many intricacies to that process. For that reason, Verve created an entire team that has spent years developing their LNP.
Designing Verve LNPs
Verve’s first LNP used as part of VERVE-101 was developed using an ionizable cationic lipid in-licensed from Acuitas.
“We were the first to say, ‘I’ve got the Acuitas ionizable lipid LNP to work in NHPs,’” said Kathiresan. “And we were the first to get [base editing] to work in mice and then non-human primates, which was published in Nature in 2021. That allowed us to take the company public. We generated a preclinical safety dataset set and an efficacy data set for that LNP that we felt very comfortable with to take forward into patients.”
Upon receiving regulatory clearance, Verve dosed its first patient with VERVE-101 in July 2022.
Shortly after that, Verve switched over to using an ionizable lipid from Novartis and developed an alternative approach to hepatic LNP uptake by using a multi-valent N-acetylgalactosamine (GalNAc) targeting ligand, which allows for uptake via the asialoglycoprotein receptor (ASGPR) pathway. This addition was intended to improve the efficacy and safety of Verve’s LNP delivery and to provide accessibility to LDL receptor-deficient patients with homozygous familial hypercholesterolemia (HoFH) and very high cholesterol.
“I think we were the first to get that to work,” said Kathiresan. “People had talked about adding GALNAC to LNP, but nobody had gotten that to work, particularly in monkeys. So, we have our own IP on GalNAc; we put it on an LNP and showed that it’s effective, which led us to our next two products—VERVE-102 and VERVE-201.”
VERVE-102 is a single-course in vivo liver-base editing medicine that aims to inactivate PCSK9, which plays a critical role in controlling blood LDL-C levels through regulation of the LDL receptor.
PCSK9 has been validated as a target through both human genetics and human pharmacology studies. As reported in The New England Journal of Medicine, one study found that adults with naturally occurring loss-of-function mutations in the PCSK9 gene had LDL-C levels that were significantly lower than in adults without a mutation, and those with a mutation had an 88% lower risk of ASCVD. Human genetic studies have also shown that carrying naturally occurring loss-of-function mutations in one or both copies of the PCSK9 gene is not associated with serious adverse health consequences.
While VERVE-102 uses the same base editor and guide RNA for PCSK9 as VERVE-101, it swaps out the LNP for the second-generation LNP that incorporates GalNAc. The internally developed GalNAc-LNP is also being used for VERVE-201 to deliver a base editor targeting another gene, ANGPTL3, to the liver.
Between VERVE-101 and VERVE-102, there’s roughly an 18-month gap in terms of development candidate selection criteria. VERVE-102 has made up some time on that lag and is gearing up for being taken into the clinic.
The serious adverse event
For the Heart-1 trial, Verve dosed 13 patients, presenting initial data in November 2023 for the first 10 patients and then for the remaining three. Six of those patients were dosed with VERVE-101 at a higher dose of 0.45 mg/kg, and the first five of them showed encouraging levels of safety and tolerability.
Shortly after dosing, the sixth patient had an elevation in ALT, known as the liver function test (LFT), and a drop in the blood platelet count. Both of these “laboratory changes” were reversible and returned to normal levels within a few days. The patient was observed in the hospital and has been doing well since being discharged, Kathiresan said.
Lost in the noise of the news of the Heart-1 pause is that, with this trial, Verve has been the first to demonstrate human proof of concept for in vivo base editing—the ability to change a single nucleotide and have a clinical effect. All of the first five patients dosed at 0.45 mg/kg are showing improvements.
“It looks like the editor and the guide are actually working as designed,” said Kathiresan. “We now have one patient who has a 73% reduction in LDL after the treatment. Then, in a couple of the patients, we have durability after the one-time treatment out to almost nine months.”
Even though the SAE patient only experienced transient and reversible “laboratory changes,” Verve elected to take a pause and consult with their Data and Safety Monitoring Board (DSMB) to better understand what happened and to figure out if there are any mitigation measures because of the early, encouraging signs of VERVE-101’s efficacy otherwise.
“The main reason to do that was that we’re seeing nice levels of efficacy, actually within our target product profile, but at the dose that we’re seeing efficacy, we’re picking up adverse events,” said Kathiresan. “The lower dose of 0.3 mg/kg that we tested (before 0.45 mg/kg) had no adverse events—but there was no efficacy. That’s a pretty narrow therapeutic index.”
Full steam ahead
Kathiresan thinks the lab safety findings are attributable to the LNP for a few reasons. First, when Verve researchers dosed animals with an LNP, they see dose-dependent increased liver function tests. Second, when they dose animals with LNPs using inactive gRNAs incapable of editing, they still see the same elevations in liver function tests. And third, there is a rapid rise in the liver function tests and a rapid drop in the platelets as well as a rapid recovery of both parameters. All of these observations overlap with the dosing time and the clearance of the lipid time.
“I’m really suggesting that it’s not the editing mechanism,” said Kathiresan. “For those three reasons, our leading hypothesis is that this is LNP-related.”
Also, Verve internally developed a process to formulate and manufacture LNPs with their own GMP facilities, but Kathiresan doesn’t think that therein lies the problem. “We don’t think [manufacturing] is the issue here,” said Kathiresan. “It’s really the underlying properties of the ionizable lipid.”
In the meantime, Kathiresan said that Verve will focus on VERVE-102, which he’s excited about not only because the LNP incorporated GalNAc cherry on top to enable reaching efficacy at even lower doses but also because the ionizable lipid has already been tested in several clinical trials, showing tolerability at efficacious doses.
The first systemically delivered CRISPR-Cas9 therapy, NTLA-2001, created by Intellia Therapeutics and Regeneron, started clinical trials in November 2020. It used a proprietary LNP (made with the same ionizable lipid from Novartis) that contains a Cas9-encoding mRNA and a sgRNA that targets the transthyretin (TTR) gene. In June 2021, Intellia published results from a successful Phase I clinical trial that included six TTR amyloidosis patients treated with NTLA-2001, demonstrating an 87% average reduction in serum TTR for the patients who received the highest dose.
In October 2023, the European Medicines Agency (EMA) named Intellia’s NTLA-2002 a priority medicine (PRIME). It is almost the same as NTLA-2001, but the guide RNA has been changed to one that targets prekallikrein (KLKB1) to treat patients with hereditary angioedema. This decision was supported by promising clinical trial data for Intellia’s NTLA-2002, published earlier this year. Intellia plans to begin recruiting patients in the United States for its global pivotal Phase III study as soon as the third quarter of 2024, pending regulatory clearance, according to financial results and highlights from Q3 2023.
“We’re trying to do something brand new that nobody’s ever done, and that kind of journey—trying to establish a first-in-class base editing treatment for cardiovascular disease —is going to come with lots of bumps on the road,” said Kathiresan. “Our job in direct development is, of course, to manage risk and have enough capital to be able to do that and to execute when you do have a bump in the road and have the right team in order to develop the safest medicine possible. I think, along all those elements, I feel very comfortable.”
Indeed, Kathiresan says that Verve has enough capital to get to another three years, which was explicitly set up in the past year or so to be able to execute against the strategy of having both the VERVE-101 and VERVE-201 programs.
“Over the last year, we articulated to the public that we’re going to pick one of these two to take forward into [Phase II] and that decision was going to come in 2025,” said Kathiresan. “So, in some sense, this is entirely on strategy. We were never going to make a decision about whether it’s going to be VERVE-101 based only on VERVE-101. We were always going to have to wait for what VERVE-102 was going to show and then pick the best of the two.”
So, whether there is reason to believe that this is the situation that Kathiresan was worried about when developing Verve’s risk management plan is irrelevant. What matters is that the patient is alive and healthy. Even better, the Verve ship isn’t just afloat; it has course-corrected and, according to its chief navigator, heading in the right direction.
The post Bittersweet Symphony: Verve’s Pause on VERVE-101 Narrows LNP-Delivery Strategy appeared first on GEN – Genetic Engineering and Biotechnology News.













