The roots of cancer run deep. Indeed, they’re usually assumed to run all the way to the blighted bits of DNA we call oncogenic mutations. But what if they don’t have to run quite that deep? What if they needn’t emerge from the genome, but from the epigenome? After all, genomically identical cells that differ epigenomically can mature toward different cell fates. Perhaps some of these fates are cancerous. The possibility seems all the more likely if we consider that many aspects of cancer susceptibility and tumorigenesis have been associated with substantial epigenomic alterations.
This possibility was explored by scientists based at the Institute of Human Genetics, CNRS, University of Montpellier. Using the familiar Drosophila model, the scientists uncovered evidence that tumors can emerge through epigenetic dysregulation leading to inheritance of altered cell fates.
Details appeared recently in Nature, in an article titled, “Transient loss of Polycomb components induces an epigenetic cancer fate.”
“[Transient] perturbation of transcriptional silencing mediated by Polycomb group proteins is sufficient to induce an irreversible switch to a cancer cell fate in Drosophila,” the article’s authors wrote. “This is linked to the irreversible derepression of genes that can drive tumorigenesis, including members of the JAK–STAT signaling pathway and zfh1, the fly homologue of the ZEB1 oncogene, whose aberrant activation is required for Polycomb perturbation-induced tumorigenesis.
These data, the authors argued, show that a reversible depletion of Polycomb proteins can induce cancer in the absence of driver mutation.
Polycomb proteins, which regulate the expression of key genes, are dysregulated in many human cancers. When these proteins are experimentally removed, the activity of the targeted genes is disrupted: some can activate their own transcription and self-maintain. When Polycomb proteins are integrated back into the cell, a subset of the genes are resistant to the proteins and remain dysregulated through cell division, allowing the cancer to continue its progression.
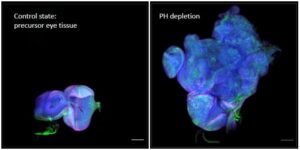
In the current study, when scientists led by Giacomo Cavalli, PhD, a research director at the Institute of Human Genetics, caused epigenetic dysregulation in the cells of Drosophila, and then restored the cells to their normal state, they found that part of the genome remains dysfunctional. This phenomenon induces a tumor state that is maintained autonomously and continues to progress, keeping in memory the cancerous status of these cells even though the signal that caused it has been restored.
“[Transient] depletion of Polycomb Repressive Complex 1 components is sufficient to switch cells into a neoplastic state that is maintained even after normal Polycomb group protein concentrations are reestablished,” the authors of the Nature article concluded. “As the same genotype can generate both a normal phenotype or a tumor depending on a transient gene regulatory modification in the absence of DNA driver mutations, we defined these tumors as epigenetically initiated cancers.”
The post Cancer Can Form without Genetic Mutations, Just Epigenetic Changes appeared first on GEN – Genetic Engineering and Biotechnology News.













